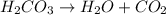Chemistry, 30.10.2019 08:31 jngonzo1226
When magnesium carbonate, mgco2, reacts with nitric acid, hno3, it forms magnesium nitrate, mg(no3)2, and carbonic acid, h2co3. carbonic acid then breaks down into water and carbon dioxide. which two types of reactions take place in this process?
a.) synthesis and decomposition
b.) single-replacement and combustion
c.) double-replacement and decomposition
d.) double-replacement and combustion

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3

Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1

Chemistry, 23.06.2019 09:30
Where are the noble gases located in the periodic table? a. in the center b. on the left side c. in the upper right corner d. on the far right side
Answers: 1

You know the right answer?
When magnesium carbonate, mgco2, reacts with nitric acid, hno3, it forms magnesium nitrate, mg(no3)2...
Questions


Business, 14.10.2021 01:00

Mathematics, 14.10.2021 01:00


Mathematics, 14.10.2021 01:00


Mathematics, 14.10.2021 01:00

Mathematics, 14.10.2021 01:00

History, 14.10.2021 01:00

Mathematics, 14.10.2021 01:00

Mathematics, 14.10.2021 01:00

Mathematics, 14.10.2021 01:00

English, 14.10.2021 01:00



Computers and Technology, 14.10.2021 01:00

Mathematics, 14.10.2021 01:00



Physics, 14.10.2021 01:00

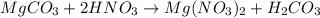 : is a double displacement reaction in which ion exchange takes place.
: is a double displacement reaction in which ion exchange takes place.