
1.If an object that stands 3 centimeters high is placed 12 centimeters in front of a plane mirror, how far from the mirror is the image located? Explain your reasoning.
2.An object with a height of 0.3 meter is placed at a distance of 0.4 meter from a concave spherical mirror. An image with a height of 0.1 meter is formed in front of the mirror. How far from the mirror is the image located?
3.When an object with a height of 0.10 meter is placed at a distance of 0.20 meter from a convex spherical mirror, the image will appear to be 0.06 meter behind the mirror. What’s the height of the image?
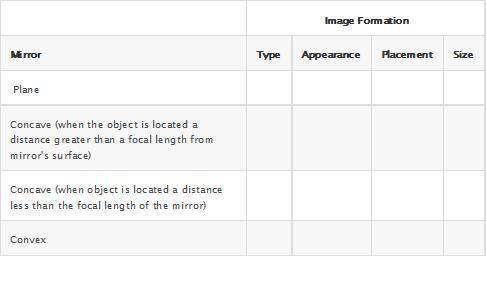
4. Compare and contrast the properties of the images formed by each mirror type in the table.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon? a balloon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 22.06.2019 13:00
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1

You know the right answer?
1.If an object that stands 3 centimeters high is placed 12 centimeters in front of a plane mirror, h...
Questions

History, 13.08.2020 01:01


Mathematics, 13.08.2020 01:01







Computers and Technology, 13.08.2020 01:01


Mathematics, 13.08.2020 01:01

Social Studies, 13.08.2020 01:01





Mathematics, 13.08.2020 01:01


Advanced Placement (AP), 13.08.2020 01:01



