
Chemistry, 03.04.2020 01:17 nathancikra
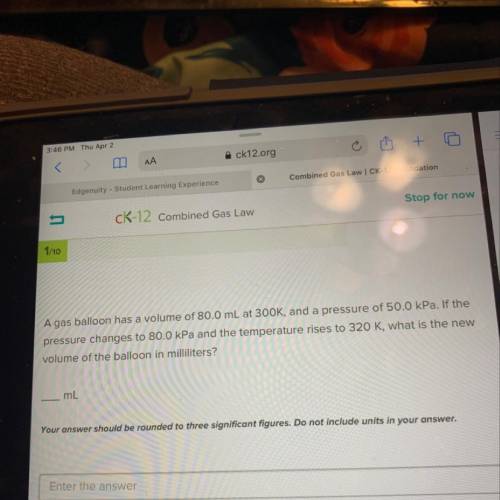
A gas balloon has a volume of 80.0 mL at 300K, and a pressure of 50.0kPa. If the pressure changes to 80.0kPa and the temperature rises to 320K, what is the new volume of the balloon in milliliters?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

You know the right answer?
A gas balloon has a volume of 80.0 mL at 300K, and a pressure of 50.0kPa. If the pressure changes to...
Questions


Physics, 03.12.2019 11:31

Social Studies, 03.12.2019 11:31

Mathematics, 03.12.2019 11:31


History, 03.12.2019 11:31

History, 03.12.2019 11:31

English, 03.12.2019 11:31


Mathematics, 03.12.2019 11:31



Biology, 03.12.2019 11:31


Health, 03.12.2019 11:31



Computers and Technology, 03.12.2019 11:31




