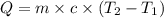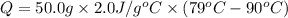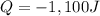Chemistry, 02.04.2020 18:24 xXwolfieplayzXx
Some thermodynamic properties of ethanol are listed in the table.
Thermodynamic Properties
Property Value
c (solid) 0.5 J/g °C
c (liquid) 1.0 J/g °C
c (gas) 2.0 J/g °C
Melting Point −114 °C
Boiling Point 78 °C
How much heat is released when 50.0 g of ethanol cools from 90 °C to 79 °C?
1,200 J
1,100 J
600 J
550 J

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1



Chemistry, 23.06.2019 22:50
When 10.g of ch3cooh is combusted in a sealed calorimeter, it releases enough energy to heat 2000. g of water from 23.5 °c to 34.3 °c. a. calculate the energy released per 10 g of ch3cooh. b. calculate the energy released per mole of ch3cooh.
Answers: 2
You know the right answer?
Some thermodynamic properties of ethanol are listed in the table.
Thermodynamic Properti...
Thermodynamic Properti...
Questions

English, 01.08.2019 04:00





Mathematics, 01.08.2019 04:00

History, 01.08.2019 04:00

Biology, 01.08.2019 04:00

English, 01.08.2019 04:00

Mathematics, 01.08.2019 04:00

English, 01.08.2019 04:00

History, 01.08.2019 04:00

Biology, 01.08.2019 04:00

Biology, 01.08.2019 04:00


Biology, 01.08.2019 04:00


Mathematics, 01.08.2019 04:00

Health, 01.08.2019 04:00

History, 01.08.2019 04:00