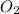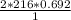2HgO(s) -> 2Hg(l) + O2(g)

Chemistry, 02.04.2020 09:43 jamesnech66
What mass of HgO is required to produce 0.692 mol of O2?
2HgO(s) -> 2Hg(l) + O2(g)


Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2
You know the right answer?
What mass of HgO is required to produce 0.692 mol of O2?
2HgO(s) -> 2Hg(l) + O2(g)
2HgO(s) -> 2Hg(l) + O2(g)
Questions


History, 05.07.2019 06:30


History, 05.07.2019 06:30


Mathematics, 05.07.2019 06:30

English, 05.07.2019 06:30


History, 05.07.2019 06:30





Health, 05.07.2019 06:30

Mathematics, 05.07.2019 06:30

English, 05.07.2019 06:30

Mathematics, 05.07.2019 06:30