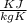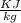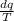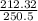Chemistry, 02.04.2020 04:26 tifftiff22
Using the relation dS = (δQ/T)int rev for the definition of entropy, calculate the change in the specific entropy of R-134a as it is heated at a constant pressure of 120 kPa from a saturated liquid to a saturated vapor. Use the tables for R-134a.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1
You know the right answer?
Using the relation dS = (δQ/T)int rev for the definition of entropy, calculate the change in the spe...
Questions


Mathematics, 14.05.2021 22:20


Mathematics, 14.05.2021 22:20






Social Studies, 14.05.2021 22:20

Mathematics, 14.05.2021 22:20


Mathematics, 14.05.2021 22:20

Mathematics, 14.05.2021 22:20

Mathematics, 14.05.2021 22:20

Mathematics, 14.05.2021 22:20



History, 14.05.2021 22:20

Mathematics, 14.05.2021 22:20