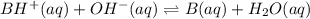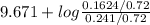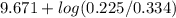Chemistry, 02.04.2020 02:30 dontcareanyonemo
A buffer that contains 0.17 M of a base, B and 0.39 M of its conjugate acid BH+, has a pH of 9.31. What is the pH after 0.02 mol of Ba(OH)2 are added to 0.72 L of the solution?

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

You know the right answer?
A buffer that contains 0.17 M of a base, B and 0.39 M of its conjugate acid BH+, has a pH of 9.31. W...
Questions

Geography, 27.01.2020 10:31

Mathematics, 27.01.2020 10:31

Social Studies, 27.01.2020 10:31


Mathematics, 27.01.2020 10:31


Mathematics, 27.01.2020 10:31



Social Studies, 27.01.2020 10:31


History, 27.01.2020 10:31

English, 27.01.2020 10:31

Mathematics, 27.01.2020 10:31






Mathematics, 27.01.2020 10:31

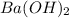 are added to 0.72 L of the solution is 9.5.
are added to 0.72 L of the solution is 9.5.
![pK_{a} + log \frac{[B]}{[BH^{+}]}](/tpl/images/0577/1202/d0a56.png)
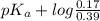
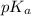 = 9.31 + 0.361
= 9.31 + 0.361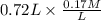
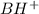 is as follows.
is as follows.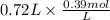
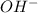 as follows.
as follows.