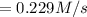Chemistry, 02.04.2020 02:03 recannon02
Consider the reaction: n2(g) 3h2(g) → 2nh3(g) suppose that a particular moment during the reaction, molecular hydrogen is reacting at a rate of 0.0687 m/s. at what rate is molecular nitrogen reacting

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 23.06.2019 07:30
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3

Chemistry, 23.06.2019 08:00
At 35.0°c and 3.00 atm pressure, a gas has a volume of 1.40 l. what pressure does the gas have at 0.00°c and a volume of 0.950 l? which equation should you use? p2= p1v1t2/t1v2what is the pressure of the gas? 3.92 atm these are the answers
Answers: 1
You know the right answer?
Consider the reaction: n2(g) 3h2(g) → 2nh3(g) suppose that a particular moment during the reaction,...
Questions

Mathematics, 24.10.2019 16:43




Mathematics, 24.10.2019 16:43

Mathematics, 24.10.2019 16:43




History, 24.10.2019 16:43

Social Studies, 24.10.2019 16:43



Biology, 24.10.2019 16:43



Physics, 24.10.2019 16:43


English, 24.10.2019 16:43

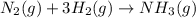
![R=-\frac{1}{1}\frac{d[N_2]}{dt}=-\frac{1}{3}\frac{d[H_2]}{dt}=\frac{1}{2}\frac{d[NH_3]}{dt}](/tpl/images/0576/9841/80d75.png)
![\frac{d[H_2]}{dt}=0.0687 M/s](/tpl/images/0576/9841/7c36a.png)
![\frac{d[N_2]}{dt}=?](/tpl/images/0576/9841/5488c.png)
![-\frac{1}{1}\frac{d[N_2]}{dt}=-\frac{1}{3}\frac{d[H_2]}{dt}](/tpl/images/0576/9841/01276.png)
![\frac{d[N_2]}{dt}=\frac{1}{3}\times 0.0687 M/s](/tpl/images/0576/9841/fd1e5.png)