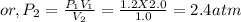A mixture of CO(g) and H2(g) is pumped into a previously evacuated 2.0 L reaction vessel. The total pressure of the reaction system is 1.2 atm at equilibrium. What will be the total equilibrium pressure of the system if the volume of the reaction vessel is reduced to 1.0 L at constant temperature

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

You know the right answer?
A mixture of CO(g) and H2(g) is pumped into a previously evacuated 2.0 L reaction vessel. The total...
Questions

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

English, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Chemistry, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01