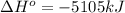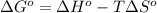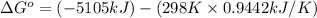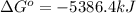Given the values of δhfo in kj/mol and so in j/mol k given below, calculate the value of δgo in kj for the reaction at 298 k: c6h12o6(s) + 6 o2(g) => 6 co2(g) + 6h2o(g) δhfo (c6h12o6) = -1,277 δhfo (co2) = -396 δhfo (h2o) = -242 so (c6h12o6(s)) = 218 so (o2(g)) = 206 so (co2(g)) = 211 so (h2o(g)) = 18

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3


You know the right answer?
Given the values of δhfo in kj/mol and so in j/mol k given below, calculate the value of δgo in kj f...
Questions

Mathematics, 27.05.2021 19:00


Mathematics, 27.05.2021 19:00

Engineering, 27.05.2021 19:00

Mathematics, 27.05.2021 19:00

Mathematics, 27.05.2021 19:00

Biology, 27.05.2021 19:00

History, 27.05.2021 19:00




English, 27.05.2021 19:00

Mathematics, 27.05.2021 19:00

Mathematics, 27.05.2021 19:00

Mathematics, 27.05.2021 19:00

Mathematics, 27.05.2021 19:00


Mathematics, 27.05.2021 19:00

Social Studies, 27.05.2021 19:00

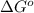 for the reaction is, -5386.4 kJ
for the reaction is, -5386.4 kJ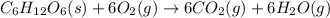
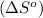 .
.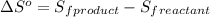
![\Delta S^o=[n_{CO_2(g)}\times \Delta S^0_{(CO_2(g))}+n_{H_2O(g)}\times \Delta S^0_{(H_2O(g))}]-[n_{C_6H_{12}O_6(s)}\times \Delta S^0_{(C_6H_{12}O_6(s))}+n_{O_2(g)}\times \Delta S^0_{(O_2(g))}]](/tpl/images/0577/2061/f660d.png)
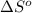 = entropy of reaction = ?
= entropy of reaction = ?![\Delta S^o=[6mole\times (211J/K.mol)+6mole\times (188.7J/K.mol]-[1mole\times (218J/K.mol)+6mole\times (206J/K.mol]](/tpl/images/0577/2061/19137.png)
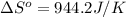
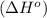 .
.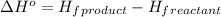
![\Delta H^o=[n_{CO_2(g)}\times \Delta H^0_{(CO_2(g))}+n_{H_2O(g)}\times \Delta H^0_{(H_2O(g))}]-[n_{C_6H_{12}O_6(s)}\times \Delta H^0_{(C_6H_{12}O_6(s))}+n_{O_2(g)}\times \Delta H^0_{(O_2(g))}]](/tpl/images/0577/2061/6b74f.png)
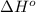 = enthalpy of reaction = ?
= enthalpy of reaction = ?![\Delta H^o=[6mole\times (-396kJ/mol)+6mole\times (-242kJ/mol]-[1mole\times (-1277kJ/mol)+6mole\times (0kJ/mol]](/tpl/images/0577/2061/b27e9.png)