
Chemistry, 01.04.2020 22:29 ashleyc2442
Given the reaction at equilibrium n2(g) + 3h2(g) ↔ 2nh3(g) increasing the concentration of N2(g) will increase the forward reaction rate due to 1. A decrease in the number of effective collisions

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
Given the reaction at equilibrium n2(g) + 3h2(g) ↔ 2nh3(g) increasing the concentration of N2(g) wil...
Questions

Mathematics, 23.10.2019 01:50

Mathematics, 23.10.2019 01:50

Mathematics, 23.10.2019 01:50


Mathematics, 23.10.2019 01:50

Mathematics, 23.10.2019 01:50



Mathematics, 23.10.2019 01:50


Geography, 23.10.2019 01:50

Mathematics, 23.10.2019 01:50

Mathematics, 23.10.2019 01:50


Social Studies, 23.10.2019 01:50




Mathematics, 23.10.2019 01:50

Mathematics, 23.10.2019 01:50

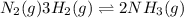 an increase in
an increase in 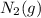 means an increase in the number of reactants.
means an increase in the number of reactants. 

