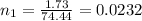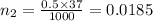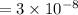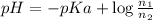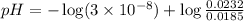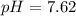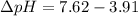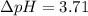Chemistry, 01.04.2020 22:32 shenzhen10
The pH of 0.50 M HClO is 3.91. Calculate the change in pH when 1.73 g of NaClO (FW = 74.44 g/mol) is added to 40 mL of 0.50 M HClO (FW = 52.46 g/mol). Ignore any changes in volume. The Ka value for HClO is 3.0 x 10-8.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
The pH of 0.50 M HClO is 3.91. Calculate the change in pH when 1.73 g of NaClO (FW = 74.44 g/mol) is...
Questions


Mathematics, 11.10.2019 05:00



Health, 11.10.2019 05:00

Mathematics, 11.10.2019 05:00

Biology, 11.10.2019 05:00




Mathematics, 11.10.2019 05:00

Mathematics, 11.10.2019 05:00

Mathematics, 11.10.2019 05:00

Mathematics, 11.10.2019 05:00

English, 11.10.2019 05:00


English, 11.10.2019 05:00


Physics, 11.10.2019 05:00