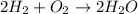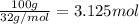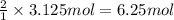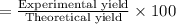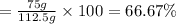Chemistry, 01.04.2020 21:45 mariarodriguezout9cj
Consider the chemical equation for the production of water: 2 H2+O2→2 H2O. If 100 grams of oxygen gas are used, what would the percent yield be if 75 g of H2O was produced? Show your work.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2


Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
You know the right answer?
Consider the chemical equation for the production of water: 2 H2+O2→2 H2O. If 100 grams of oxygen ga...
Questions

History, 22.09.2019 19:30

Mathematics, 22.09.2019 19:30



Chemistry, 22.09.2019 19:30

Mathematics, 22.09.2019 19:30

Mathematics, 22.09.2019 19:30

Mathematics, 22.09.2019 19:30

Mathematics, 22.09.2019 19:30

Mathematics, 22.09.2019 19:30


Mathematics, 22.09.2019 19:30


Mathematics, 22.09.2019 19:30

Mathematics, 22.09.2019 19:30



Business, 22.09.2019 19:30

Chemistry, 22.09.2019 19:30

History, 22.09.2019 19:30