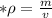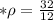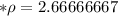Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
A piece of aluminum has a volume of 12 cm3 and a mass of 32 g. What is its density?...
Questions

Mathematics, 25.02.2021 06:50

Mathematics, 25.02.2021 06:50

Mathematics, 25.02.2021 06:50


Social Studies, 25.02.2021 06:50

Mathematics, 25.02.2021 06:50

Spanish, 25.02.2021 06:50

Mathematics, 25.02.2021 06:50

Chemistry, 25.02.2021 06:50




English, 25.02.2021 06:50

Spanish, 25.02.2021 06:50

Mathematics, 25.02.2021 07:00

Chemistry, 25.02.2021 07:00

Mathematics, 25.02.2021 07:00

Mathematics, 25.02.2021 07:00


Mathematics, 25.02.2021 07:00