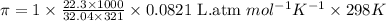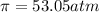Chemistry, 01.04.2020 19:31 kjjackson012002
What is the osmotic pressure of a solution made from 22.3 g of methanol (MM = 32.04 g/mol) that was added to water to make 321 mL of solution at 25.0 °C?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
what is the density of an object that has a mass of 10 g and a volumeof 5 ml? a. 0.5 g/ mlb. 2 g/mlc. 15 g/ mld. 50 g/ ml
Answers: 1

Chemistry, 21.06.2019 18:00
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1
You know the right answer?
What is the osmotic pressure of a solution made from 22.3 g of methanol (MM = 32.04 g/mol) that was...
Questions

Social Studies, 13.01.2020 03:31

Mathematics, 13.01.2020 03:31



English, 13.01.2020 03:31





Arts, 13.01.2020 03:31

Mathematics, 13.01.2020 03:31

English, 13.01.2020 03:31



Mathematics, 13.01.2020 03:31





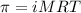
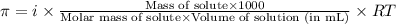
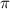 = osmotic pressure of the solution = ?
= osmotic pressure of the solution = ?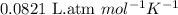
![25^oC=[273+25]=298K](/tpl/images/0575/9078/6a9f9.png)