
Chemistry, 01.04.2020 19:20 taylor5384
What quantity, in moles, of oxygen is consumed when 717.4 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g)? H2(g) + O2(g) → H2O(l); ΔH° = –285.8 kJ

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 23.06.2019 03:30
Ineed pls urgent 1-20 in order and fully detail step my step.
Answers: 1

You know the right answer?
What quantity, in moles, of oxygen is consumed when 717.4 kJ of energy is evolved from the combustio...
Questions

Mathematics, 19.05.2021 02:30

Mathematics, 19.05.2021 02:30

Mathematics, 19.05.2021 02:30

Mathematics, 19.05.2021 02:30

History, 19.05.2021 02:30

Mathematics, 19.05.2021 02:30



English, 19.05.2021 02:30

Computers and Technology, 19.05.2021 02:30

Mathematics, 19.05.2021 02:30



English, 19.05.2021 02:30






Computers and Technology, 19.05.2021 02:30

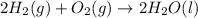
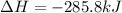
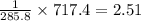 moles
moles

