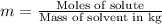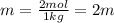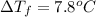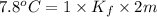Chemistry, 01.04.2020 18:36 kwarwick0915
Two moles of a nonelectrolyte solute are dissolved in 1 kg of an unknown solvent. the solution freezes at 7.8°c below its normal freezing point. what is the molal freezing-point constant of the unknown solvent? suggest a possible identity of the solvent.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
Two moles of a nonelectrolyte solute are dissolved in 1 kg of an unknown solvent. the solution freez...
Questions



History, 16.02.2021 01:10




Mathematics, 16.02.2021 01:20

Mathematics, 16.02.2021 01:20

English, 16.02.2021 01:20

Health, 16.02.2021 01:20

Mathematics, 16.02.2021 01:20

Biology, 16.02.2021 01:20

Computers and Technology, 16.02.2021 01:20

Mathematics, 16.02.2021 01:20

English, 16.02.2021 01:20




Mathematics, 16.02.2021 01:20

History, 16.02.2021 01:20

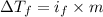
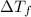 =depression in freezing point =
=depression in freezing point = 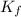 = freezing point constant
= freezing point constant