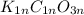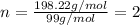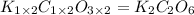Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1
You know the right answer?
A sample of a compound contains 7.89 g potassium, 2.42 g carbon, and 9.69 g oxygen. Determine the em...
Questions

Mathematics, 04.02.2021 14:00


Mathematics, 04.02.2021 14:00



Medicine, 04.02.2021 14:00


Mathematics, 04.02.2021 14:00

Mathematics, 04.02.2021 14:00

History, 04.02.2021 14:00


Mathematics, 04.02.2021 14:00



Advanced Placement (AP), 04.02.2021 14:00

Mathematics, 04.02.2021 14:00

Social Studies, 04.02.2021 14:00

English, 04.02.2021 14:00


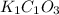
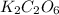
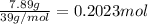

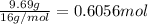
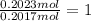
![\frac{0.2017 mol}{0.2017 mol}=1Oxygen : [tex]\frac{0.6056 mol}{0.2017 mol}=3](/tpl/images/0575/6268/097b3.png)