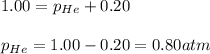The gas that a diver breathes must be maintained at a partial pressure of 0.20 atm of oxygen. More oxygen could poison the diver, and less would lead to suffocation. The total pressure, however, must be equal to the external pressure to avoid collapsing the lungs. A special valve is used to equalize the pressure inside the diver's lungs with the external pressure by adding helium gas. The valve also maintains oxygen levels by using Dalton's Law. If the total pressure in the cylinder is 1.00 atm, what is the pressure of the helium?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Measuring which physical property is most likely to produce the most precise results when trying to identify a substance
Answers: 1

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

You know the right answer?
The gas that a diver breathes must be maintained at a partial pressure of 0.20 atm of oxygen. More o...
Questions

Mathematics, 24.05.2021 16:10

Mathematics, 24.05.2021 16:10


Computers and Technology, 24.05.2021 16:10


Mathematics, 24.05.2021 16:10

Mathematics, 24.05.2021 16:10


History, 24.05.2021 16:10



English, 24.05.2021 16:20

Computers and Technology, 24.05.2021 16:20




Biology, 24.05.2021 16:20



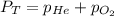
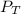 = 1.00 atm
= 1.00 atm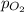 = 0.20 atm
= 0.20 atm