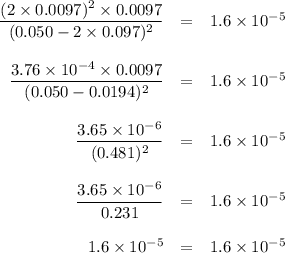Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
2NOCI (g)
→ 2NO(g) + Cl2 (g)
Problem:
Initially
the above reaction contains<...
→ 2NO(g) + Cl2 (g)
Problem:
Initially
the above reaction contains<...
Questions





History, 19.04.2021 01:00


Mathematics, 19.04.2021 01:00




Mathematics, 19.04.2021 01:00

Mathematics, 19.04.2021 01:00

Mathematics, 19.04.2021 01:00

Biology, 19.04.2021 01:00

History, 19.04.2021 01:00

Spanish, 19.04.2021 01:00



English, 19.04.2021 01:00

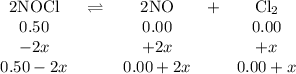
![K_{\text{c}} = \dfrac{\text{[NO]$^{2}$[Cl$_{2}$]}}{\text{[NOCl]}^{2}} = \dfrac{(2x)^{2}(x)}{(0.50 - 2x)^{2}} = 1.6 \times 10^{-5}\\\\4x^{3} = 1.6 \times 10^{-5}(0.50 - 2x)^{2}\\x^{3} = 4.0 \times 10^{-6}(0.50 - 2x)^{2}](/tpl/images/0575/0584/940ed.png)
![x = \sqrt [3] {4.0 \times 10^{-6}(0.50)^{2}} = 0.010](/tpl/images/0575/0584/0f7f4.png)
![x = \sqrt [3] {4.0 \times 10^{-6}(0.50 - 2\times 0.010)^{2}} = 0.0097](/tpl/images/0575/0584/39746.png)
![x = \sqrt [3] {4.0 \times 10^{-6}(0.50 - 2\times 0.0097)^{2}} = 0.0097](/tpl/images/0575/0584/5e7db.png)