
Chemistry, 01.04.2020 01:04 ericadawn2852
William measures a test tube and finds that the mass of the test tube is 5g. He places the lone reactant in the test tube and finds the mass to be 12.5g. He carries out a reaction by heating the test tube. At the end of the reaction, he measures the mass of the test tube containing the lone product and finds it to have a mass of 9.3g. What is the percent yield for this experiment if he expected to produce 5g of product?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
William measures a test tube and finds that the mass of the test tube is 5g. He places the lone reac...
Questions





Mathematics, 21.07.2019 01:50

Mathematics, 21.07.2019 01:50


History, 21.07.2019 01:50









SAT, 21.07.2019 01:50



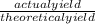 x 100
x 100 x 100
x 100

