
The rate of disappearance of HBr in the gas phase reaction 2 HBr(g) → H2(g) + Br2(g) is 0.140 M s-1 at 150°C. The rate of appearance of Br2 is M s-1. The rate of disappearance of HBr in the gas phase reaction 2 HBr(g) → H2(g) + Br2(g) is 0.140 M s-1 at 150°C. The rate of appearance of Br2 is M s-1.
a. 0.0700
b. 1.28
c. 0.0196
d. 0.280
e. 0.374

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
Lithium diisopropylamide [(ch3)2ch]2nli, referred to as lda, enjoys many uses as a strong base in synthetic organic chemistry. it is customarily prepared by the reaction of diisopropylamine [(ch3)2ch]2nh with butyllithium. draw the products of the reactions in the appropriate boxes and select the acid, base, conjugate acid, and conjugate base. be sure to answer all parts.
Answers: 2

Chemistry, 21.06.2019 18:00
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1
You know the right answer?
The rate of disappearance of HBr in the gas phase reaction 2 HBr(g) → H2(g) + Br2(g) is 0.140 M s-1...
Questions

Mathematics, 28.01.2021 04:40




Mathematics, 28.01.2021 04:40


Physics, 28.01.2021 04:40

Mathematics, 28.01.2021 04:40

English, 28.01.2021 04:40

History, 28.01.2021 04:40

Mathematics, 28.01.2021 04:40

Chemistry, 28.01.2021 04:40


Mathematics, 28.01.2021 04:40



Mathematics, 28.01.2021 04:40

Mathematics, 28.01.2021 04:40

Mathematics, 28.01.2021 04:40

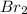 is
is 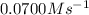
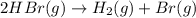
![-\frac{1d[HBr]}{2dt}Rate in terms of appearance of [tex]H_2](/tpl/images/0573/9318/b52fb.png) =
= ![\frac{1d[H_2]}{dt}](/tpl/images/0573/9318/06e14.png)
![\frac{1d[Br_2]}{dt}](/tpl/images/0573/9318/f0ecb.png)
![-\frac{1d[HBr]}{2dt}=\frac{d[H_2]}{dt}=\frac{d[Br_2]}{dt}](/tpl/images/0573/9318/40849.png)
![-\frac{1d[HBr]}{dt}=0.140Ms^{-1}](/tpl/images/0573/9318/2b756.png)
![\frac{1d[Br_2]}{dt}=-\frac{1d[HBr]}{2dt}=\frac{1}{2}\times 0.140=0.0700Ms^{-1}](/tpl/images/0573/9318/cf50e.png)


