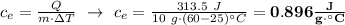Chemistry, 01.10.2019 19:50 justinhk10
Exactly 313.5 j will raise the temperature of 10.0 g of a metal from 25.0 c to 60.0 c. what is the specific heat of the metal?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
Exactly 313.5 j will raise the temperature of 10.0 g of a metal from 25.0 c to 60.0 c. what is the s...
Questions

Biology, 08.12.2020 23:20




Mathematics, 08.12.2020 23:20




English, 08.12.2020 23:20

Mathematics, 08.12.2020 23:20

Mathematics, 08.12.2020 23:20

Biology, 08.12.2020 23:20

English, 08.12.2020 23:20

Mathematics, 08.12.2020 23:20

World Languages, 08.12.2020 23:20

Mathematics, 08.12.2020 23:20




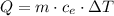 we can clear specific heat:
we can clear specific heat: