Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2
You know the right answer?
0.50 mol A, 0.60 mol B, and 0.90 mol C are reacted according to the following reaction
A + 2B...
A + 2B...
Questions


Mathematics, 28.09.2019 07:30

Mathematics, 28.09.2019 07:30


History, 28.09.2019 07:30

Social Studies, 28.09.2019 07:30

Mathematics, 28.09.2019 07:30


Mathematics, 28.09.2019 07:30

Mathematics, 28.09.2019 07:30

Biology, 28.09.2019 07:30

Health, 28.09.2019 07:30

English, 28.09.2019 07:30



English, 28.09.2019 07:30

Biology, 28.09.2019 07:30

Mathematics, 28.09.2019 07:30

English, 28.09.2019 07:30

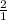 =
= 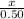
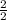 =
= 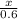
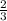 =
=