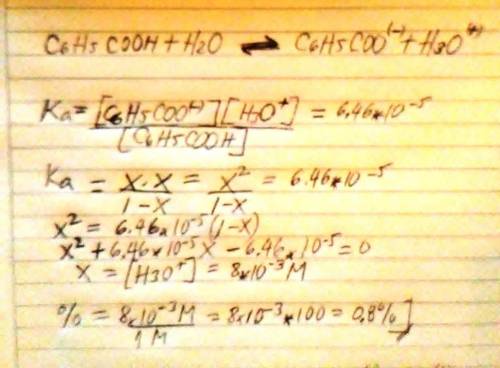The acid ionization constant for benzoic acid (C6H5COOH) is 6.46 × 10−5 . Compare the percent ionization of 1.00 M benzoic acid in water with its percent ionization in 0.500 M sodium benzoate solution. Support your comparison with calculations similar to those in Model 1.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
The acid ionization constant for benzoic acid (C6H5COOH) is 6.46 × 10−5 . Compare the percent ioniza...
Questions

History, 12.10.2019 18:30


Business, 12.10.2019 18:30




Biology, 12.10.2019 18:30


Physics, 12.10.2019 18:30

Social Studies, 12.10.2019 18:30



Mathematics, 12.10.2019 18:30


Mathematics, 12.10.2019 18:30

History, 12.10.2019 18:30

Biology, 12.10.2019 18:30