
The rate constant for a first-order reaction is 0.54 s-1. What is the half-life of this reaction if the initial concentration is 0.54 M? The rate constant for a first-order reaction is 0.54 s-1. What is the half-life of this reaction if the initial concentration is 0.54 M? 4.7 s 0.49 s 1.0 s 1.3 s 1.8 s

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2
You know the right answer?
The rate constant for a first-order reaction is 0.54 s-1. What is the half-life of this reaction if...
Questions

Mathematics, 04.12.2020 22:30

Mathematics, 04.12.2020 22:30


Social Studies, 04.12.2020 22:30


Health, 04.12.2020 22:30

Mathematics, 04.12.2020 22:30


Biology, 04.12.2020 22:30


Mathematics, 04.12.2020 22:30

Mathematics, 04.12.2020 22:30



Mathematics, 04.12.2020 22:30

Mathematics, 04.12.2020 22:30

SAT, 04.12.2020 22:30

Mathematics, 04.12.2020 22:30

Mathematics, 04.12.2020 22:30

Biology, 04.12.2020 22:30

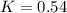
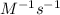
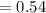 M
M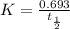
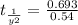
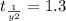 sec
sec

