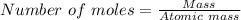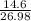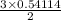Chemistry, 31.03.2020 04:37 chantianabess36
Sulfuric acid dissolves aluminum metal according to the following reaction: 2Al(s)+3H2SO4(aq)→Al2(SO4)3(aq)+3H2 (g)2Al(s)+3H2SO4(aq)→Al2(SO4)3(aq)+ 3H2(g) Suppose you wanted to dissolve an aluminum block with a mass of 14.6 gg . Part A What minimum mass of H2SO4H2SO4 would you need? Express your answer in grams.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
Sulfuric acid dissolves aluminum metal according to the following reaction: 2Al(s)+3H2SO4(aq)→Al2(SO...
Questions

Mathematics, 11.11.2020 01:50

History, 11.11.2020 01:50

Mathematics, 11.11.2020 01:50

English, 11.11.2020 01:50



Mathematics, 11.11.2020 01:50


Chemistry, 11.11.2020 01:50



Mathematics, 11.11.2020 01:50


Mathematics, 11.11.2020 01:50

Biology, 11.11.2020 01:50


Chemistry, 11.11.2020 01:50


Mathematics, 11.11.2020 01:50

Law, 11.11.2020 01:50