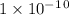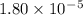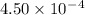Chemistry, 31.03.2020 04:19 jessicaflower277
Given the following acids and their Ka values: Phenol, C6H5OH Ka = 1.00 × 10-10 Acetic acid, CH3CO2H Ka = 1.80 × 10-5 Nitrous acid, HNO2 Ka = 4.50 × 10-4 What is the order of increasing base strength for C6H5O−, CH3COO−, and NO2−? Group of answer choices

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2


Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1
You know the right answer?
Given the following acids and their Ka values: Phenol, C6H5OH Ka = 1.00 × 10-10 Acetic acid, CH3CO2H...
Questions




English, 24.04.2021 18:20


History, 24.04.2021 18:20

Mathematics, 24.04.2021 18:20

English, 24.04.2021 18:20

Social Studies, 24.04.2021 18:20

Chemistry, 24.04.2021 18:20


Mathematics, 24.04.2021 18:20

Health, 24.04.2021 18:20


English, 24.04.2021 18:20



History, 24.04.2021 18:20


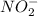 <
< 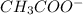 <
< 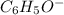
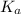 value is a measure of acidic strength. The larger the
value is a measure of acidic strength. The larger the