
Chemistry, 31.03.2020 03:21 cathydaves
The compound carbon suboxide, C3O2, is a gas at room temperature. Use the data supplied to calculate the heat of formation of carbon suboxide. (Data: 2CO(g) + C(s) → C3O2(g) ΔH° = 127.3 kJ/mol and: ΔHf° of CO(g) = –110.5 kJ/mol)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2
You know the right answer?
The compound carbon suboxide, C3O2, is a gas at room temperature. Use the data supplied to calculate...
Questions





Mathematics, 05.02.2020 06:53


Mathematics, 05.02.2020 06:54


Mathematics, 05.02.2020 06:54


Mathematics, 05.02.2020 06:54





Social Studies, 05.02.2020 06:54

Chemistry, 05.02.2020 06:54



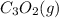 is -92.7 kJ/mol
is -92.7 kJ/mol![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f_{(product)}]-\sum [n\times \Delta H^o_f_{(reactant)}]](/tpl/images/0572/6547/72c39.png)
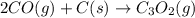
![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(C_3O_2(g))})]-[(2\times \Delta H^o_f_{(CO(g))})+(1\times \Delta H^o_f_{(C(s))})]](/tpl/images/0572/6547/3be78.png)
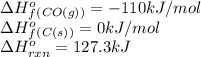
![127.3=[(1\times \Delta H^o_f_{(C_3O_2(g))})]-[(2\times (-110))+(1\times (0))]\\\\\Delta H^o_f_{(C_3O_2(g))}=-92.7kJ/mol](/tpl/images/0572/6547/5dacc.png)


