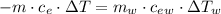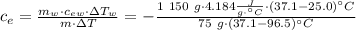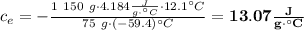Chemistry, 22.09.2019 00:30 Arianahinton9856
Achemist mixes 75.0 g of an unknown substance at 96.5°c with 1,150 g of water at 25.0°c. if the final temperature of the system is 37.1°c, what is the specific heat capacity of the substance? use 4.184 j / g °c for the specific heat capacity of water.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3


Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
Achemist mixes 75.0 g of an unknown substance at 96.5°c with 1,150 g of water at 25.0°c. if the fina...
Questions

English, 12.12.2020 16:20

Chemistry, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20


Mathematics, 12.12.2020 16:20


Mathematics, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20

English, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20




Mathematics, 12.12.2020 16:20

English, 12.12.2020 16:20

History, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20

Spanish, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20