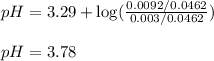Chemistry, 31.03.2020 01:36 abbypoletick
When a 26.3 mL sample of a 0.465 M aqueous nitrous acid solution is titrated with a 0.461 M aqueous barium hydroxide solution, what is the pH after 19.9 mL of barium hydroxide have been added

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
When a 26.3 mL sample of a 0.465 M aqueous nitrous acid solution is titrated with a 0.461 M aqueous...
Questions

Mathematics, 31.01.2021 20:40

Mathematics, 31.01.2021 20:40




Biology, 31.01.2021 20:40


Mathematics, 31.01.2021 20:40

Mathematics, 31.01.2021 20:40

English, 31.01.2021 20:40


English, 31.01.2021 20:40

English, 31.01.2021 20:40

Computers and Technology, 31.01.2021 20:40



Mathematics, 31.01.2021 20:40



English, 31.01.2021 20:40

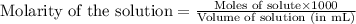 .......(1)
.......(1)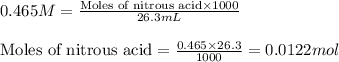
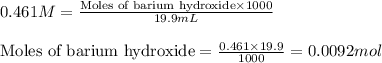
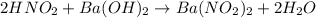
![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0572/3345/e4eea.png)
![pH=pK_a+\log(\frac{[NO_2^-]}{[HNO_2]}](/tpl/images/0572/3345/c621f.png)
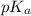 = negative logarithm of acid dissociation constant of nitrous acid = 3.29
= negative logarithm of acid dissociation constant of nitrous acid = 3.29![[NO_2^-]=\frac{0.0092}{0.0462}](/tpl/images/0572/3345/4843c.png)
![[HNO_2]=\frac{0.003}{0.0462}](/tpl/images/0572/3345/5be58.png)