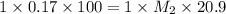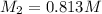Chemistry, 31.03.2020 01:05 bjpvrpow74wq
To standardize a solution of NaOH before using it in a titration of an unknown acid, you dissolve 3.56 grams of potassium hydrogen phthalate (KHP) into 100 mL of H2O. You then titrate the KHP with your sodium hydroxide solution and reach the endpoint after adding 20.9 mL of NaOH. What is the molarity of your sodium hydroxide solution? (Molecular Mass of KHP = 204.22 g/mol)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1

Chemistry, 23.06.2019 08:00
How does the digestive system interact with the circulatory system? a. messages sent as electrical impulses from the digestive system are transported throughout the body by the circulatory system. b. nutrients taken in and broken down by the digestive system are carried to various parts of the body by the circulatory system. c. nutrients and gases are absorbed by organs in the circulatory system. then, they are transported to all parts of the body by organs in the digestive system. d. oxygen and carbon dioxide are exchanged by organs in the digestive system, and the gases are carried to the rest of the body by the circulatory system.
Answers: 2
You know the right answer?
To standardize a solution of NaOH before using it in a titration of an unknown acid, you dissolve 3....
Questions

Chemistry, 11.12.2019 06:31

Mathematics, 11.12.2019 06:31

Mathematics, 11.12.2019 06:31


History, 11.12.2019 06:31

Mathematics, 11.12.2019 06:31

History, 11.12.2019 06:31

Mathematics, 11.12.2019 06:31

Chemistry, 11.12.2019 06:31

Mathematics, 11.12.2019 06:31

History, 11.12.2019 06:31

Mathematics, 11.12.2019 06:31

History, 11.12.2019 06:31


Mathematics, 11.12.2019 06:31


English, 11.12.2019 06:31


Mathematics, 11.12.2019 06:31


 = volume of solution in ml = 100 ml
= volume of solution in ml = 100 ml

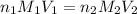
 = molarity of KHP solution = 0.17 M
= molarity of KHP solution = 0.17 M = volume of KHP solution = 100 ml
= volume of KHP solution = 100 ml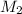 = molarity of NaOH solution = ?
= molarity of NaOH solution = ? = volume of NaOH solution = 20.9 ml
= volume of NaOH solution = 20.9 ml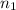 = valency of KHP = 1
= valency of KHP = 1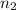 = valency of NaOH = 1
= valency of NaOH = 1