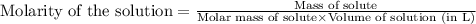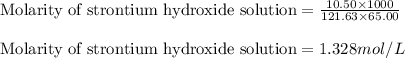Chemistry, 31.03.2020 00:51 smarty5187
A strontium hydroxide solution is prepared by dissolving 10.50 g of Sr(OH)2 in water to make 65.00 mL of solution. What is the molarity of this solution? Express your answer to four significant figures and include the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
A strontium hydroxide solution is prepared by dissolving 10.50 g of Sr(OH)2 in water to make 65.00 m...
Questions


Mathematics, 25.05.2020 04:59


Biology, 25.05.2020 04:59

Chemistry, 25.05.2020 04:59






Mathematics, 25.05.2020 04:59


English, 25.05.2020 04:59

Chemistry, 25.05.2020 04:59





Mathematics, 25.05.2020 04:59