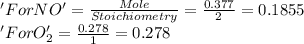Chemistry, 31.03.2020 00:46 kateferguson9852
Nitrogen dioxide is produced by combustion in an automobile engine. For the following reaction, 0.377 moles of nitrogen monoxide are mixed with 0.278 moles of oxygen gas. What is the formula for the limiting reagent? What is the maximum amount of nitrogen dioxide that can be produced?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1
You know the right answer?
Nitrogen dioxide is produced by combustion in an automobile engine. For the following reaction, 0.37...
Questions

Mathematics, 16.04.2020 02:04









History, 16.04.2020 02:04

History, 16.04.2020 02:04



Mathematics, 16.04.2020 02:04




English, 16.04.2020 02:04

Mathematics, 16.04.2020 02:05