How many moles of \ce{CO2}COX
2
will be produced from 72.0 \text{ g}72.0...


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:10
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3
You know the right answer?
Questions

Biology, 21.04.2020 17:59

Social Studies, 21.04.2020 17:59


Social Studies, 21.04.2020 17:59








Social Studies, 21.04.2020 17:59






Mathematics, 21.04.2020 17:59

Physics, 21.04.2020 17:59

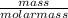
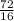 = 4.5moles
= 4.5moles 


