
Chemistry, 31.03.2020 00:13 electrofy456
The solid XY decomposes into gaseous X and Y: XY(s) ⇌ X(g) + Y(g) Kc = 4.1(at 0C) If the reaction is carried out in a 1-L container, which initial amounts of X and Y result in the formation of solid XY?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
The solid XY decomposes into gaseous X and Y: XY(s) ⇌ X(g) + Y(g) Kc = 4.1(at 0C) If the reaction is...
Questions

Mathematics, 08.09.2021 21:50


Mathematics, 08.09.2021 21:50

Mathematics, 08.09.2021 21:50

Biology, 08.09.2021 21:50

Mathematics, 08.09.2021 21:50

Physics, 08.09.2021 21:50



Mathematics, 08.09.2021 21:50

English, 08.09.2021 21:50

English, 08.09.2021 21:50




Geography, 08.09.2021 21:50

Mathematics, 08.09.2021 21:50

Mathematics, 08.09.2021 21:50


Mathematics, 08.09.2021 21:50

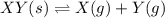 Kp = 4.1 (at 0°C)
Kp = 4.1 (at 0°C)![Q=[X][Y]](/tpl/images/0572/0334/b3caf.png)
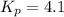
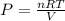
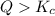 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.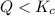 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.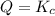 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.

