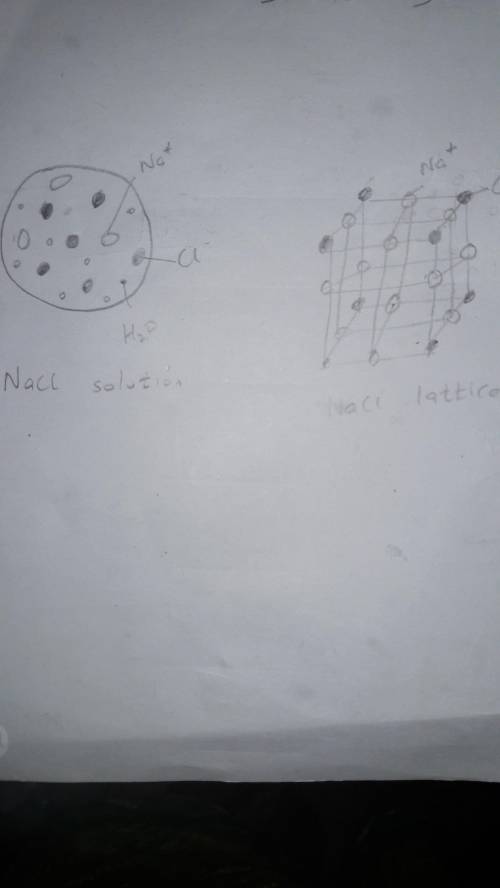
Classify the following as either a heterogeneous or homogeneous mixture, and explain your answers. a. orange juice b. tap water 2. a. What are substances called whose water solutions conduct electricity? b. Why does a salt solution conduct electricity? c. Why does a sugarwater solution not conduct electricity? 3. Make a drawing of the particles in an NaCl solution to show why this solution conducts electricity. Make a drawing of the particles in an NaCl crystal to show why pure salt does not conduct. 4. Describe one way to prove that a mixture of sugar and water is a solution and that a mixture of sand and water is not a solution. 5. Name the solute and solvent in the following: a. 14-karat gold b. corn syrup c. carbonated, or sparkling, water Critical Thinking 6. ANALYZING INFORMATION If you allow a container of sea water to sit in the sun, the liquid level gets lower and lower, and

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 07:20
Part b: study of equilibrium on solubility: mg(oh)2(s) ⇌ mg2+(aq) + 2 oh–(aq) cloudy clear (pink) 7. a. b. 8. a. b. 9. 10. 11. 12. when adding concentrated hydrochloric acid, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 7a. you should indicate which ion was added to or removed from the equilibrium mixture. when adding edta, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 8a. you should indicate which ion was added to or removed from the equilibrium mixture. upon heating in which direction is the equilibrium shifting? upon cooling in which direction is the equilibrium shifting? is the forward reaction a. endothermic explain your answers to questions 9, 10, and 11. (l) left (r) right (l) left (r) right b. exothermic
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
You know the right answer?
Classify the following as either a heterogeneous or homogeneous mixture, and explain your answers. a...
Questions


Social Studies, 15.12.2020 20:30


Social Studies, 15.12.2020 20:30


Mathematics, 15.12.2020 20:30


Mathematics, 15.12.2020 20:30



Chemistry, 15.12.2020 20:30

Mathematics, 15.12.2020 20:30

Mathematics, 15.12.2020 20:30


Biology, 15.12.2020 20:30


English, 15.12.2020 20:30



Geography, 15.12.2020 20:30