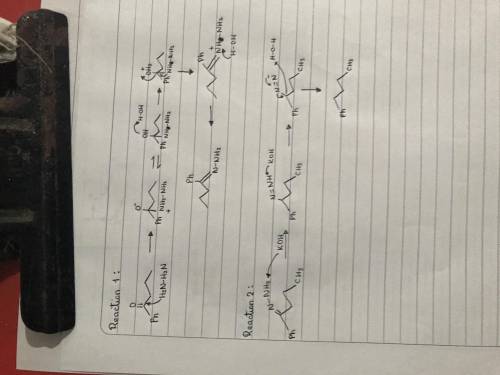
Chemistry, 30.03.2020 23:46 abieber4328
The Wolff-Kishner reaction involves the reaction of an aldehyde/ketone with hydrazine in the presence of KOH. The process is useful for converting an aldehyde or ketone into an alkane. The reaction involves formation of a hydrazone, followed by base-catalyzed double-bond migration, loss of N2 gas to give a carbanion, and protonation to give the alkane. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1
You know the right answer?
The Wolff-Kishner reaction involves the reaction of an aldehyde/ketone with hydrazine in the presenc...
Questions


Mathematics, 23.06.2019 13:00




Chemistry, 23.06.2019 13:00




Mathematics, 23.06.2019 13:00

English, 23.06.2019 13:00

Chemistry, 23.06.2019 13:00





History, 23.06.2019 13:00

Physics, 23.06.2019 13:00