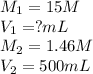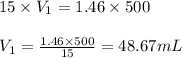Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
The ammonia solution that is purchased for a stockroom has a molarity of 15 M. Determine the volume...
Questions



Social Studies, 10.12.2020 21:40



Mathematics, 10.12.2020 21:40


Chemistry, 10.12.2020 21:40





Mathematics, 10.12.2020 21:40

Mathematics, 10.12.2020 21:40

Business, 10.12.2020 21:40

Chemistry, 10.12.2020 21:40



Mathematics, 10.12.2020 21:40

Mathematics, 10.12.2020 21:40

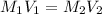
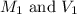 are the molarity and volume of the stock ammonia solution
are the molarity and volume of the stock ammonia solution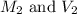 are the molarity and volume of diluted ammonia solution
are the molarity and volume of diluted ammonia solution