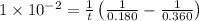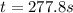Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3
You know the right answer?
A reaction that is second-order in one reactant has a rate constant of 1 × 10–2 L/mol · s. If the in...
Questions

Mathematics, 29.08.2020 22:01

Business, 29.08.2020 22:01



Mathematics, 29.08.2020 22:01








Computers and Technology, 29.08.2020 22:01


Business, 29.08.2020 22:01

English, 29.08.2020 22:01

Mathematics, 29.08.2020 22:01



![k=\frac{1}{t}\left (\frac{1}{[A]}-\frac{1}{[A]_o}\right)](/tpl/images/0571/7035/5ea71.png)
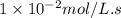
![[A]_o](/tpl/images/0571/7035/9caf5.png) = Initial concentration = 0.360 mol/L
= Initial concentration = 0.360 mol/L