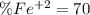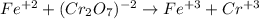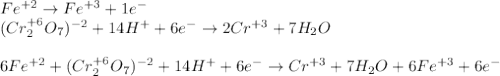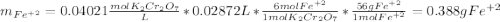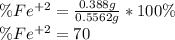Chemistry, 30.03.2020 23:08 GreenHerbz206
An iron ore sample weighing 0.5562 g is dissolved HCl (aq), and the iron is obtained as Fe2 in solution. This solution is then titrated with 28.72 mL of 0.04021 M K2Cr2O7 (aq). What is the percent by mass iron in the ore sample

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Can you reduce your impairment by drinking coffee or cold showers? true or false?
Answers: 3

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2
You know the right answer?
An iron ore sample weighing 0.5562 g is dissolved HCl (aq), and the iron is obtained as Fe2 in solut...
Questions

Social Studies, 07.04.2020 00:34

Mathematics, 07.04.2020 00:34

History, 07.04.2020 00:34

Mathematics, 07.04.2020 00:34


Mathematics, 07.04.2020 00:34

Mathematics, 07.04.2020 00:34


Chemistry, 07.04.2020 00:34



Mathematics, 07.04.2020 00:34

History, 07.04.2020 00:34




Social Studies, 07.04.2020 00:34

Geography, 07.04.2020 00:34

Mathematics, 07.04.2020 00:34