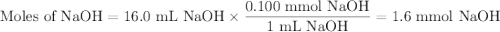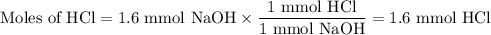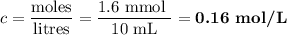Base your answer on the information below and on your knowledge of chemistry.
A NaOH(aq)...

Chemistry, 30.03.2020 22:52 gghkooo1987
Base your answer on the information below and on your knowledge of chemistry.
A NaOH(aq) solution with a pH value of 13 is used to determine the molarity of an HCl(aq) solution. A 10.0-mL sample of the HCl(aq) is exactly neutralized by 16.0 mL of 0.100 M NaOH(aq). During this laboratory activity, appropriate safety equipment was used and safety procedures were followed.
Determine the pH value of a solution that has a H+(aq) ion concentration 10 times greater than the original NaOH(aq) solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
Questions

Mathematics, 19.07.2019 07:10







Mathematics, 19.07.2019 07:10

Mathematics, 19.07.2019 07:10

Mathematics, 19.07.2019 07:10

Law, 19.07.2019 07:10




Advanced Placement (AP), 19.07.2019 07:10

Law, 19.07.2019 07:10

English, 19.07.2019 07:10