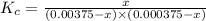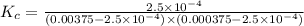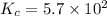3.0 mL of 0.02 M Fe(NO3)3 solution is mixed with 3.0 mL of 0.002 M NaNCS and diluted to the mark with HNO3 in 10 mL volumetric flask. The blood-red [Fe(NCS)]2+ ion that forms has an equilibrium molar concentration of 2.5*10-4 mol/L as determined from the calibration plot. Calculate the Kc for [Fe(NCS)]2+ formation. Assume the volumes are additive.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 07:00
If you used the method of initial rates to obtain the order for no2, predict what reaction rates you would measure in the beginning of the reaction for initial concentrations of 0.200 m, 0.100 m, & 0.050 m no2.
Answers: 3
You know the right answer?
3.0 mL of 0.02 M Fe(NO3)3 solution is mixed with 3.0 mL of 0.002 M NaNCS and diluted to the mark wit...
Questions


Mathematics, 20.09.2019 22:30






Mathematics, 20.09.2019 22:30

Mathematics, 20.09.2019 22:30

Mathematics, 20.09.2019 22:30

Mathematics, 20.09.2019 22:30



Mathematics, 20.09.2019 22:30

History, 20.09.2019 22:30


Mathematics, 20.09.2019 22:30


Mathematics, 20.09.2019 22:30

Mathematics, 20.09.2019 22:30

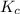 for
for ![[Fe(NCS)]^{2+}](/tpl/images/0571/7802/c2420.png) formation is
formation is  .
.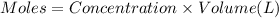
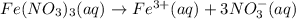
![[Fe(NO_3)_3]=0.02 M=[Fe^{3+}]](/tpl/images/0571/7802/da55e.png)
![[Fe^{3+}]=0.02 M](/tpl/images/0571/7802/a8178.png)
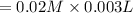
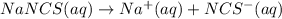
![[NaNCS]=0.002 M=[NCS^-]](/tpl/images/0571/7802/ed7be.png)
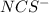 ion =
ion = ![[NCS^{-}]=0.002 M](/tpl/images/0571/7802/84149.png)
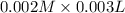
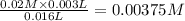
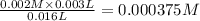
![Fe^{3+}+NCS^-\rightleftharpoons [Fe(NCS)]^{2+}](/tpl/images/0571/7802/1aeb7.png)
![[Fe(NCS)]^{2+}=x=2.5\times 10^{-4} M](/tpl/images/0571/7802/1a7a9.png)
![K_c=\frac{[[Fe(NCS)]^{2+}]}{[Fe^{3+}][NCS^-]}](/tpl/images/0571/7802/4616c.png)