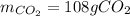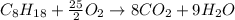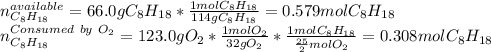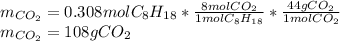Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 66. g of octane is mixed with 123. g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3


Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

You know the right answer?
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . S...
Questions


Mathematics, 16.02.2021 06:10


Mathematics, 16.02.2021 06:10

Mathematics, 16.02.2021 06:10

English, 16.02.2021 06:10

Mathematics, 16.02.2021 06:10

Mathematics, 16.02.2021 06:10

Mathematics, 16.02.2021 06:10




Social Studies, 16.02.2021 06:10

History, 16.02.2021 06:10

Mathematics, 16.02.2021 06:10


Mathematics, 16.02.2021 06:10

Chemistry, 16.02.2021 06:10

English, 16.02.2021 06:10

Biology, 16.02.2021 06:10