Chemistry, 30.03.2020 22:32 savannahvargas512
Determine the limiting reactant in a mixture containing 139 g of Se, 431 g of Cl2, and 110 g of H2O. Calculate the maximum mass (in grams) of selenic acid, H2SeO4, that can be produced in the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1



You know the right answer?
Determine the limiting reactant in a mixture containing 139 g of Se, 431 g of Cl2, and 110 g of H2O....
Questions

Chemistry, 03.10.2021 15:40

Mathematics, 03.10.2021 15:40

Chemistry, 03.10.2021 15:40

Mathematics, 03.10.2021 15:40

Advanced Placement (AP), 03.10.2021 15:40

Chemistry, 03.10.2021 15:40

Social Studies, 03.10.2021 15:40


English, 03.10.2021 15:40


English, 03.10.2021 15:40

Mathematics, 03.10.2021 15:40


World Languages, 03.10.2021 15:40



English, 03.10.2021 15:40



Mathematics, 03.10.2021 15:40

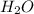
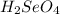 produced is, 220.4 grams.
produced is, 220.4 grams.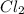 = 431 g
= 431 g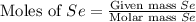
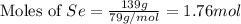
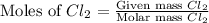
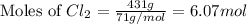
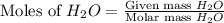
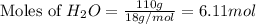
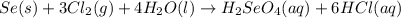
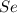
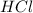 react with
react with 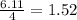 moles of
moles of 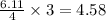 moles of
moles of 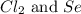 are excess reagent because the given moles are greater than the required moles and
are excess reagent because the given moles are greater than the required moles and