
Chemistry, 30.03.2020 22:42 OGrant18075
Following the instructions in your lab manual, you have titrated a 25.00 mL sample of 0.0100 M KIO3 with a solution of Na2S2O3 of unknown concentration. The endpoint was observed to occur at 16.50 mL. 1. How many moles of KIO3 were titrated? Show work! 2. How many moles of Na2S2O3 did this require?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1
You know the right answer?
Following the instructions in your lab manual, you have titrated a 25.00 mL sample of 0.0100 M KIO3...
Questions



Social Studies, 09.04.2021 14:20

Mathematics, 09.04.2021 14:20

Biology, 09.04.2021 14:20

Social Studies, 09.04.2021 14:20




English, 09.04.2021 14:20


English, 09.04.2021 14:20

English, 09.04.2021 14:30



Engineering, 09.04.2021 14:30

English, 09.04.2021 14:30

Computers and Technology, 09.04.2021 14:30

Mathematics, 09.04.2021 14:30

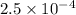 moles
moles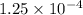 moles
moles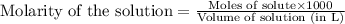
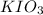 solution = 0.0100 M
solution = 0.0100 M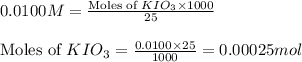
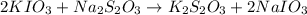
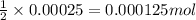 of sodium thiosulfate
of sodium thiosulfate

