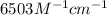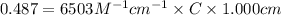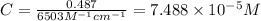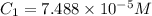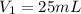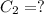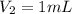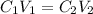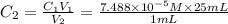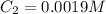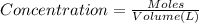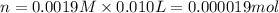An unknown amount of a compound with a molecular mass of 264.37 g/mol is dissolved in a 10 mL volumetric flask. A 1.00 mL aliquot of this solution is transferred to a 25 mL volumetric flask, and enough water is added to dilute to the mark. The absorbance of this diluted solution at 327 nm is 0.487 in a 1.000 cm cuvette. The molar absorptivity for this compound at 327 nm is ϵ 327 = 6503 M^(−1) cm^(−1).
(a) What is the concentration of the compound in the cuvette?
(b) What is the concentration of the compound in the 10-mL flask?
(c) How many milligrams of the compound were used to make the 10-mL solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1


Chemistry, 24.06.2019 00:00
\problem page what kind of intermolecular forces act between a dichlorine monoxide molecule and a chloroacetylene molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1
You know the right answer?
An unknown amount of a compound with a molecular mass of 264.37 g/mol is dissolved in a 10 mL volume...
Questions

Mathematics, 05.05.2020 05:50


Mathematics, 05.05.2020 05:50

Mathematics, 05.05.2020 05:50

Social Studies, 05.05.2020 05:50

Mathematics, 05.05.2020 05:50

Mathematics, 05.05.2020 05:50

Mathematics, 05.05.2020 05:50

Mathematics, 05.05.2020 05:51





Engineering, 05.05.2020 05:51



English, 05.05.2020 05:51


Chemistry, 05.05.2020 05:51

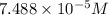 is the concentration of the compound in the cuvette.
is the concentration of the compound in the cuvette.
 = molar absorptivity of this solution =
= molar absorptivity of this solution =