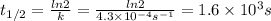Chemistry, 30.03.2020 21:50 karenjjensen
The rate constant, k, for a first-order reaction is equal to 4.3 × 10-4 s-1. What is the half-life for the reaction? The rate constant, k, for a first-order reaction is equal to 4.3 × 10-4 s-1. What is the half-life for the reaction? 1.9 × 103 s 1.2 × 103 s 1.6 × 103 s 3.0 × 10-4 s

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3


Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
The rate constant, k, for a first-order reaction is equal to 4.3 × 10-4 s-1. What is the half-life f...
Questions


Biology, 17.01.2020 02:31




English, 17.01.2020 02:31

Mathematics, 17.01.2020 02:31

Chemistry, 17.01.2020 02:31

History, 17.01.2020 02:31

History, 17.01.2020 02:31

Mathematics, 17.01.2020 02:31


Spanish, 17.01.2020 02:31





Mathematics, 17.01.2020 02:31

Mathematics, 17.01.2020 02:31

![rate=k \times [A]^{m}](/tpl/images/0571/4200/f39c7.png)