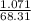Chemistry, 30.03.2020 22:13 FreddyNoTalKing
A 1.897g sample of Mg(HCO3)2 was heated and decomposed. When the sample
cooled, it weighed 1.071g. What is the % yield of this reaction?
Mg(HCO3)2 (s) + CO2 (g) + H2O (g) + MgCO2 (s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1


Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
A 1.897g sample of Mg(HCO3)2 was heated and decomposed. When the sample
cooled, it weighed 1.0...
cooled, it weighed 1.0...
Questions


Biology, 21.07.2019 11:00


Mathematics, 21.07.2019 11:00

English, 21.07.2019 11:00






Mathematics, 21.07.2019 11:00


Chemistry, 21.07.2019 11:00

Physics, 21.07.2019 11:00

History, 21.07.2019 11:00

Mathematics, 21.07.2019 11:00


Biology, 21.07.2019 11:00

History, 21.07.2019 11:00

Social Studies, 21.07.2019 11:00

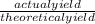 x 100
x 100