
Chemistry, 30.03.2020 21:43 sindy35111
1. Based on the appearance of your reaction in the beaker, which reagent do you think was consumed and which reagent had some left over? The aluminum was consumed, and copper was left over as seen by the reddish particles. 2. If 5.0 g of iron metal is reacted with 15.0 g of Cl2 gas, how many grams of ferric chloride will form? About 14.52 grams will form. 3. For the reaction above the amount of ferric chloride obtained in the lab was 9.15 grams. Calculate the percent yield. The percent yield would be around 63.02%. 4. What are some reasons for obtaining a percent yield of less than 100 percent? Factors such as the reactants not reacting completely, human error in the experiment, the reactants might have too large of a surface area for reaction, multiple reactions occurring within an experiment, temperature, etc.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
1. Based on the appearance of your reaction in the beaker, which reagent do you think was consumed a...
Questions

History, 01.08.2019 05:30




History, 01.08.2019 05:30

Biology, 01.08.2019 05:30






Biology, 01.08.2019 05:30

English, 01.08.2019 05:30







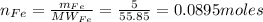
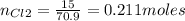
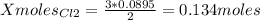
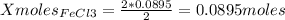
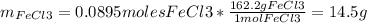
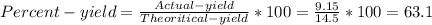 %
%

